As explained on cell metabolism intro, during catabolism, larger molecules are broken into smaller ones and the energy this "molecule-busting" releases is immediately repackaged into "energy-transfer" molecules - high-energy molecules that are used for moment-to-moment energy-transfers (and NOT for long-term energy storage - fats and carbohydrates are used for that).
The two most important of these energy-transfer molecules are Adenosine triphosphate (ATP) and Nicotinamide adenine dinucleotide phosphate (NADPH).
Both are formed by energy released in the oxidation-reduction reactions of catabolism. ATP is used to power all kinds of things - from basic cellular functions to anabolism to the movements of muscle cells. NADPH is used as a "reducing agent" during anabolism. (1)
NADPH covers
NADPH and "reducing agents".
This page covers how ATP is made and used.

ATP is sometimes thought of as "energy-currency" because cells are constantly converting energy into ATP and then "cashing" ATP and using the energy that was stored in it to do work.
It is formed by joining one molecule called a "phosphate group" (Pi) to another molecule called "ADP" (this process consumes energy and thus requires some kind of effort) (2).
People do most of their ATP-making during cellular respiration. Plants make ATP during photosynthesis but use most of that ATP to make the glucose that they then turn into ATP during cellular respiration.
Yeast and many other creatures can make enough ATP to survive with just fermentation - which is much less efficient than respiration.
ATP is useful for driving chemical reactions because of the mobility of the phosphate group (Pi).
With the help of enzymes and hydrolysis (breaking chemical bonds via reactions with water - see hydrolysis), the phosphate group (Pi) can be removed from ATP and transferred to some other molecule (call it "Calmo") and the result (besides turning the ATP molecule back into an ADP molecule) is to turn molecule Calmo into a new, less stable molecule "Nutto".
Because Nutto is less stable than Calmo, it is more chemically reactive and will react in ways Calmo would've never dreamed of reacting. (2)
When Nutto reacts (doing whatever work that was desired in the first place), the phosphate group (Pi) is freed and ready to be used again to make a new ATP molecule. The molecules used to make ATP (ADP and Pi) are constantly recylced. (2)
This process of transferring a phosphate molecule from a molecule of ATP to some other molecule is used by cells to power all kinds of things from active transport (forcing things in and out of the cell) to the molecular-bonding of anabolism to the movement of muscle cells. (3)
As an example of ATP in action, let's look at anabolism (forming larger molecules out of smaller ones). The main steps are also shown in the diagrams on the left side of the page.
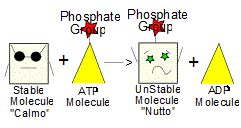
1. Suppose you want to get two molecules, "Calmo" and "Hapless" to bond to form a new molecule "Togetherness" but the chemical reaction would have to consume energy in order for it to happen (it won't happen "on its own", aka: "spontaneously"). Where do we get the needed energy?
2. ATP! With the help of enzymes and hydrolysis, a phosphate group (Pi) can be transferred from a molecule of ATP onto Calmo, turning Calmo into a new, less stable molecule "Nutto".
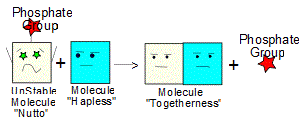
3. Since it is so unstable, molecule Nutto is also more reactive and will now readily bond with molecule "Hapless", forming a shiny, new molecule Togetherness. In the process, the phosphate group (Pi) is freed and so Togetherness is made up of Calmo and Hapless - which is what we wanted way back in step 1.. (4)
The amount of ATP your cells make and use each day is amazing.
About half of the food you eat is converted into ATP and then back into the various cellular actions that ultimately power and sustain your body.
It is estimated that the average adult human (here given a weight of 70kg/154lbs) goes through about their own body weight in ATP each day by constantly recycling the approximately 50 grams (.1 pound) of ATP/ADP that his or her body contains! (5)
So, the next time you move a muscle, you might think about all the little ATP molecules that make your muscles move even though they don't know you or have any idea what you are doing or care in the least about you walking over to say 'hi' to so and so who is on the other side of the street and who you haven't seen in some time...
Fantastic though ATP is, it can't do everything. The molecule-building reactions of anabolism require a "reducing agent"> and that is what NADPH is for.
If this page has been a helpful
please recommend it with Google+ below:
1 High-energy biomolecules are discussed throughout the second edition of the textbook
Biochemistry by Garrett and Grisham, currently (6/20/2011) available online at
Heidi
In section "3.6 · The High-Energy Biomolecules", they are introduced and we learn,
"A small family of universal biomolecules mediates the flow of energy from exergonic reactions to the energy-requiring
processes of life. These molecules are the reduced coenzymes and the high-energy phosphate compounds." In that section,
they also point out that these two molecules are not used for long-term storage, but rather,
"They are transient forms of stored energy, meant to carry energy from point to point,
from one enzyme system to another, in the minute-to-minute existence of the cell."
2 This footnote is linked to three topics - (1) the making of ATP, (2) the using of ATP, (3) the recycling of ATP.
I found a useful overview
of ATP in Biology, sixth edition by Campbell and Reece, Copyright 2002 by Pearson Education, Inc. The
relevant section is "Chapter 6: An Introduction to Metabolism"
(1) That making ATP out of ADP and Pi uses energy released from catabolism is on page 96;
(2) That hydrolysis removes a Pi from an ADP is on page 94;
That Enzymes then aid in transferring the Pi to the target molecule which turns it into another, less
stable and therefore more reactive molecule is on page 95;
(3) That the cells keep making new ATP out of ADP and Pi is on page 95.
3 the textbook (Biology) and section cited in footnote #2 also provided this information.
The
energy-needs of the cell and the fact that ATP is usually "the immediate source of energy that powers cellular work" is
on page 94. Additionally, on page 95 there is a diagram showing how ATP is used in anabolism and on page 149 of
"Chapter 8: Membrane Structure and Function" of the same book, there is a diagram showing how ATP is used in active transport
(pumping molecules across the cell membrane that would not otherwise go the direction you want them to go).
4 An example of how ATP is used in anabolism is found in the same section of the textbook (Biology)
cited in footnote #2.
On page 95, there is a diagram showing how ATP can be used to get the molecules glutamic acid and
ammonia to form glutamine. This diagram served as the basis for my explanation of how ATP can power anabolism as well as
my diagrams on the same theme.
5 The percentage of consumed calories that becomes ATP as well as the calculation that the average adult
human (70 kg) consumes close to their own body weight in ATP each day and that they only contain 50 grams of ATP/ADP within them
is all in section "3.8 The Daily Human Requirement of ATP" of:
the second edition of the textbook Biochemistry by Garrett and Grisham, currently (6/20/2011) available online at
Heidi.